By clicking “Accept All Cookies” you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Privacy Policy
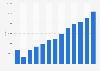
The U.S. plasmid DNA manufacturing market size was estimated at USD 815.32 million in 2024 and is projected to be worth around USD 4,310.78 million by 2034, growing at a CAGR of 18.11% from 2025 to 2034.
| Industry Worth | Details |
| Market Size in 2025 | USD 987.36 Million |
| Market Size by 2034 | USD 4,310.78 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 18.11% |
Driven by America's leading role in biotechnological innovation and sophisticated gene therapy research, the plasmid DNA production market in the United States is both robust and undergoing significant expansion. The country has a strong biopharmaceutical sector that is progressively funding the creation of DNA-based vaccines and gene therapies. Central to these therapeutic and preventive techniques, plasmid DNA manufacturing highlights its significance in ongoing clinical investigation and product development. These research and development efforts the United States’ dedication to increasing high-quality plasmid DNA output for commercial and clinical applications, as evidenced by a spike in strategic alliances, facility expansions, and technology licensing agreements among American biotech companies.
High costs of gene therapy are hampering the growth of the market
High costs of gene therapy especially those related to plasmid DNA production pose a significant restriction for market expansion. These costs result from the complexity of production processes, including purification of plasmid DNA synthesis and viral vector, as well as adherence to strict quality criteria. Costs are further inflated by specialized cleanroom facilities, difficulties in scaling up production, and the need for trained personnel. Beyond production, the high-risk, long, and costly clinical trial procedure also adds major risk. These interacting elements mean that gene therapies are expensive, so reducing accessibility and acceptance, particularly in cost-sensitive healthcare systems, which therefore limits the wider growth of the plasmid DNA production industry.
Emerging prospects in many therapeutic fields
Emerging prospects in many therapeutic fields, including oncology, neurological disorders, and infectious diseases, are driving the growth of the plasmid DNA production market. Gene and mRNA-based therapies rely on plasmid DNA to provide therapeutic genes and to serve as starting material for mRNA injections. In rare diseases and neurological disorders, oncology supports DNA vaccination and directed gene delivery, which helps rectify genetic flaws. Furthermore, increasing its relevance is its function in synthetic biology and DNA vaccines. These developments are fueling demand for high-quality plasmid DNA and opening up other development possibilities for the production sector.
Published by Ajit Bansod
| Subsegment | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 | 2030 | 2031 | 2032 | 2033 | 2034 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Viral Vectors | - | - | - | - | - | - | - | - | - | - | - |
| Plasmid DNA | - | - | - | - | - | - | - | - | - | - | - |
| Non-Viral | - | - | - | - | - | - | - | - | - | - | - |
| Electroporation | - | - | - | - | - | - | - | - | - | - | - |
| Lipid/Polymer | - | - | - | - | - | - | - | - | - | - | - |
| Nanoparticles | - | - | - | - | - | - | - | - | - | - | - |
| Others | - | - | - | - | - | - | - | - | - | - | - |
| Subsegment | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 | 2030 | 2031 | 2032 | 2033 | 2034 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Therapy | - | - | - | - | - | - | - | - | - | - | - |
| DNA Vaccines | - | - | - | - | - | - | - | - | - | - | - |
| Immunotherapy | - | - | - | - | - | - | - | - | - | - | - |
| Others | - | - | - | - | - | - | - | - | - | - | - |
| Subsegment | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 | 2030 | 2031 | 2032 | 2033 | 2034 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Infectious Disease | - | - | - | - | - | - | - | - | - | - | - |
| Genetic Disorder | - | - | - | - | - | - | - | - | - | - | - |
| Cancer | - | - | - | - | - | - | - | - | - | - | - |
To get full access to our Market Insights, you need a Professional Account or a Business Suite.
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.

You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.

Our customers work more efficiently and benefit from