By clicking “Accept All Cookies” you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Privacy Policy
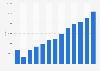
The Europe plasmid DNA manufacturing market size was calculated at USD 588.6 million in 2024 and is predicted to attain around USD 3,115.75 million by 2034, expanding at a CAGR of 18.13% from 2025 to 2034.
| Industry Worth | Details |
| Market Size in 2025 | USD 712.8 Million |
| Market Size by 2034 | USD 3115.75 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 18.13% |
The European plasmid DNA production sector is growing considerably as a result of rising funding for vaccine research and gene therapy. Leading biotech and pharmaceutical firms call this area home; they use plasmid DNA for therapeutic development and clinical studies. European nations are promoting gene treatment answers for rare and long-term diseases, therefore framing plasmid DNA as a basic element in this wave of invention. Government-funded research support and regulatory backing from agencies such as the EMA are improving the region’s capacity in advanced therapy medicinal products (ATMPs). Rising focus on biopharmaceutical self-sufficiency is spurring funding for regional plasmid DNA producing facilities in important countries included in the continent such as Germany, France, and the UK. Strategic partnerships are also rising, so positioning Europe to be pivotal in the global supply chain for plasmid DNA.
Strict regulatory challenges
Complicated regulatory challenges provide significant constraints on the plasmid DNA (pDNA) production sector. Particularly for smaller companies, strict compliance with cGMP standards—especially for clinical-grade DNA increases manufacturing costs and postpones time-to-market. Great technical obstacles are ensuring purity, eliminating pollutants, and keeping uniform quality at scale. Regulatory agencies like the FDA set strict approval standards calling for great testing and paperwork. Growing concern about the occurrence of antibiotic resistance genes in plasmids also compounds regulatory pressure. These obstacles restrict pDNA-based treatments’ more widespread use in clinical and commercial contexts by impeding scalability and marketability.
Research and development opportunities
The fast rise of the plasmid DNA production sector presents significant possibilities for research and development as well as invention in academia. Optimizing production methods to enhance scalability and cost-efficiency, especially via developments in cell culture and downstream purification technologies, Key market possibilities would be provided. Rising uses in gene editing, synthetic biology, and personalized medicine are also opening fresh commercial possibilities. Moreover, meeting increasing regulatory expectations depends on improving quality control systems. R&D is being hastened by partnerships among biotech companies, CROs, and academic institutions. Furthermore, boosting market potential are the emerging manufacturing platforms, including suspension cell systems and viral vector technologies, and the developments they represent.
The viral vector segment led the Europe plasmid DNA manufacturing market with a major revenue share in 2024. The plasmid DNA production market is primarily driven by the widespread use of adeno-associated viral vectors in cell-based gene therapies, therefore dominating the viral vector segment. Their strong market position is strengthened by their necessity in modern therapeutic plans as well as their capacity to include enormous transgenes and sustain high-yield output.
The gene therapy segment led the Europe plasmid DNA manufacturing market with a remarkable revenue share in 2024. Still, the most common application area, gene therapy, is in high demand for therapies aimed at genetic and hereditary disorders. Constant innovation in gene delivery methods and safer therapeutic platforms is driving adoption and revealing continuous development possibilities.
Infectious diseases dominated the market in 2024. At present, infectious diseases account for the majority of demand for plasmid DNA since they play a key role in DNA-based vaccination’s ability to elicit strong immune responses. Cancer, on the other hand, looks to be a much higher-potential development area since plasmid DNA will become essential to create new cancer treatments employing gene-modified vectors.
Published by Ajit Bansod
| Subsegment | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 | 2030 | 2031 | 2032 | 2033 | 2034 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Viral Vectors | - | - | - | - | - | - | - | - | - | - | - |
| Plasmid DNA | - | - | - | - | - | - | - | - | - | - | - |
| Non-Viral | - | - | - | - | - | - | - | - | - | - | - |
| Electroporation | - | - | - | - | - | - | - | - | - | - | - |
| Lipid/Polymer | - | - | - | - | - | - | - | - | - | - | - |
| Nanoparticles | - | - | - | - | - | - | - | - | - | - | - |
| Others | - | - | - | - | - | - | - | - | - | - | - |
| Subsegment | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 | 2030 | 2031 | 2032 | 2033 | 2034 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Therapy | - | - | - | - | - | - | - | - | - | - | - |
| DNA Vaccines | - | - | - | - | - | - | - | - | - | - | - |
| Immunotherapy | - | - | - | - | - | - | - | - | - | - | - |
| Others | - | - | - | - | - | - | - | - | - | - | - |
| Subsegment | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 | 2030 | 2031 | 2032 | 2033 | 2034 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Infectious Disease | - | - | - | - | - | - | - | - | - | - | - |
| Genetic Disorder | - | - | - | - | - | - | - | - | - | - | - |
| Cancer | - | - | - | - | - | - | - | - | - | - | - |
To get full access to our Market Insights, you need a Professional Account or a Business Suite.
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.

You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.

Our customers work more efficiently and benefit from